
A common misconception has been lingering in the galvanizing industry for decades:
"The thicker the zinc coating, the better!" - Customer
Many manufacturers who are eager to reduce their production cost and the prices for their customers know, a thicker zinc coating does not always result in better product performance. However, it is often difficult to explain this to the customer who often doesn't completely understand why. And, since a handful of end use products do benefit from a thicker coating, selling products with low zinc weight can sometimes become a struggle.
So, which is it now? Does zinc coating thickness or zinc weight really matter?
In general, it depends on the end-use. Some end-users do have special requirements that need a thick coating. For example, in environments such as construction work, scaffolding tubes are more frequently exposed to contact than, say, those used for fencing, structural support, etc. Scaffolding tubes are exposed to abrasion, which comes from handling together with sand, concrete and similar substances used in construction work. Sand particles can have a higher hardness than zinc, which then can scratch the surface. Concrete and other substances during construction can have a negative impact on galvanized surfaces, due to their acidic, or in concrete's case alkaline properties.
One of the reasons why more zinc weight is still being associated with better, longer product performance is the ongoing effort of hot dip galvanizers trying to retain customers and sell their products. This is mainly because the typical hot dip galvanizing process is not as efficient as continuous galvanizing processes when producing thin zinc coatings (< 200 g/m²) leading to manufacturers choosing to apply more zinc than the end-use application may require.
Excessive zinc application can be evidenced by Zn/fe alloy layers in the following photomicrograph.
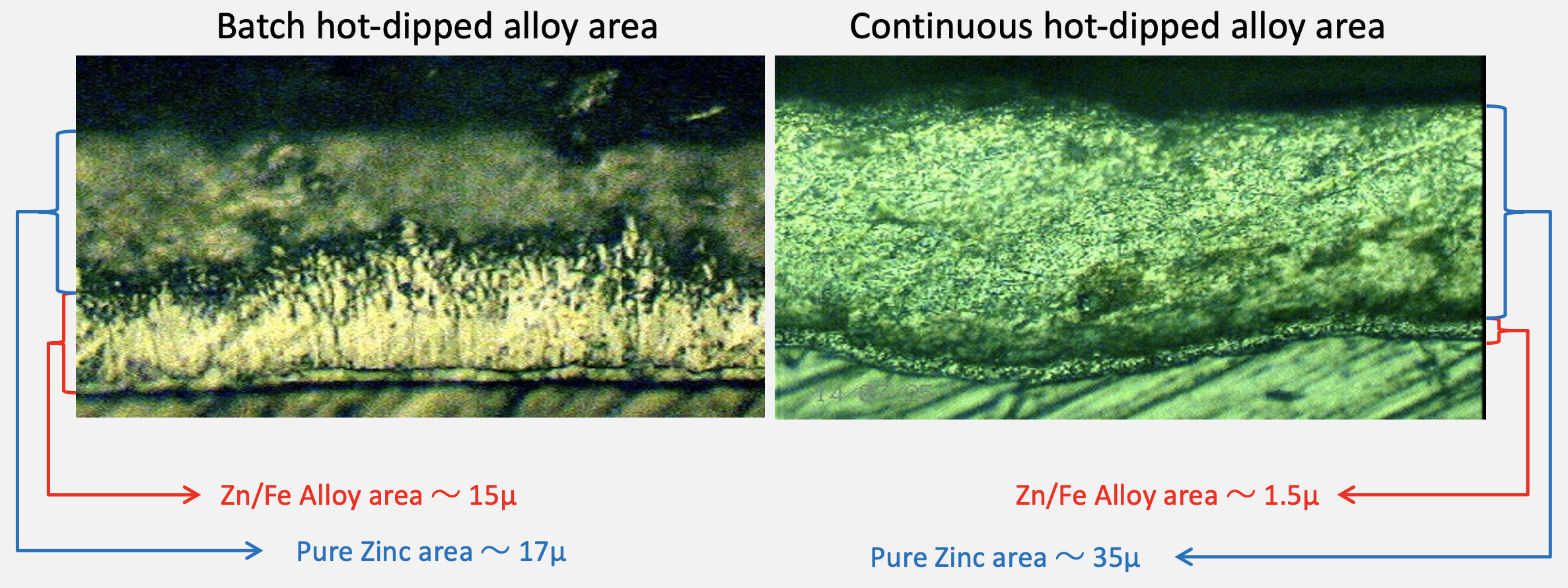
Simplified, to achieve a thick zinc coating, the time that steel is being emerged in molten zinc needs to be extended. However, the longer this period lasts, the more reaction will happen between the zinc and the steel itself, forming and growing a zinc/iron alloy layer. However, the Zn/Fe alloy layer has inferior corrosion protection properties compared to zinc.
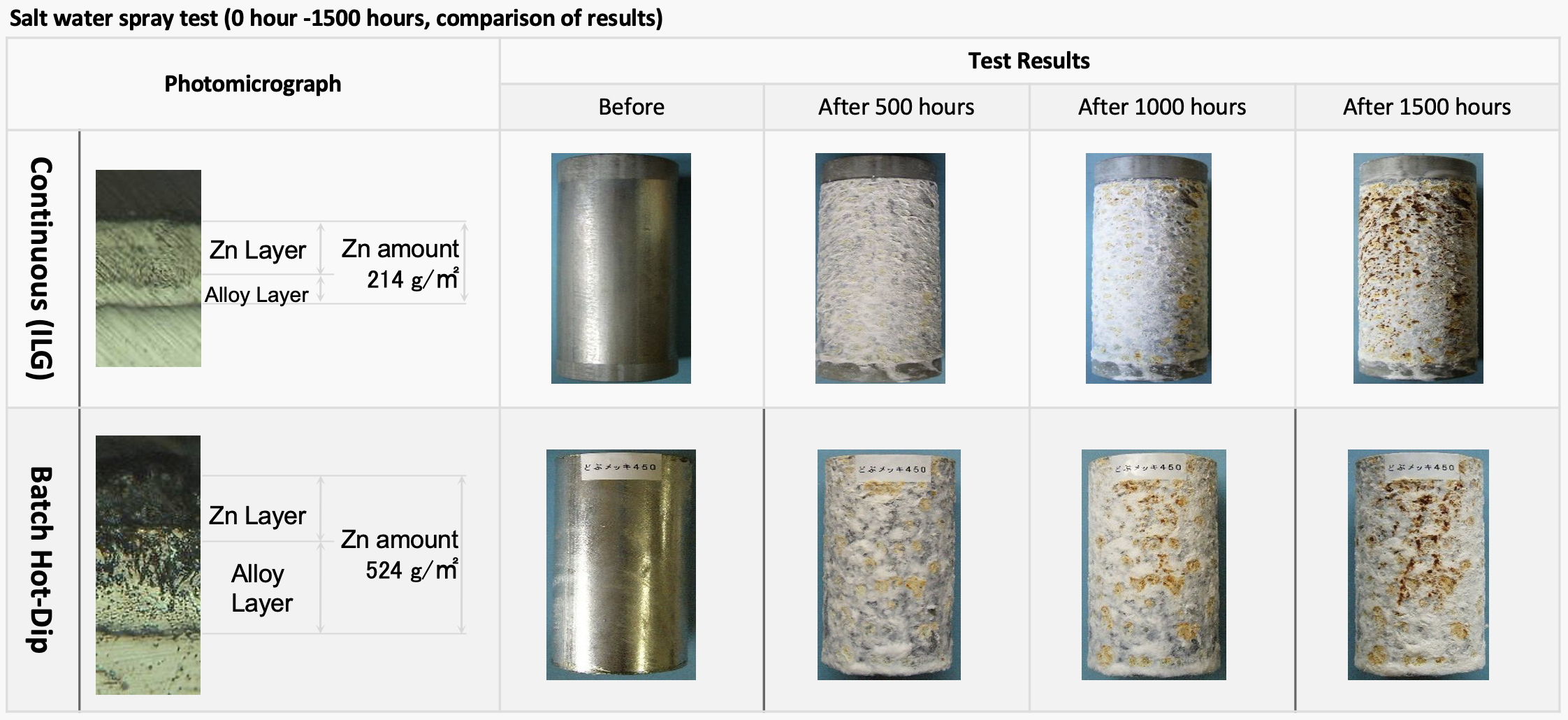
A salt water spray test simulates natural corrosion in a short period of time, what else would have taken years. The above salt water spray test results conducted by one of our customers show that the lifetime of products galvanized by batch hot dip with a coating thickness of 524g/m² is about the same as that of an in-line galvanized product with a zinc coating thickness of 214g/m² In other words, the thickness of the pure zinc layer of the product significantly improves the protective properties of the galvanized surface to natural corrosion.
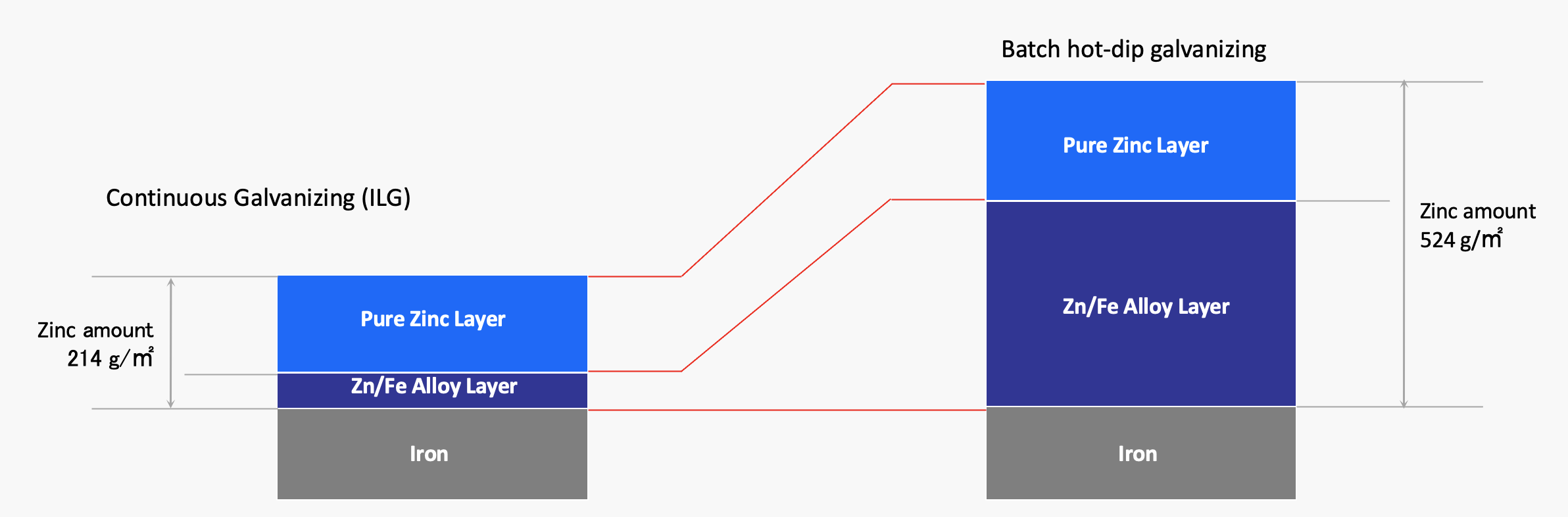
Summary
While some products last longer with thicker zinc coating, it is often due to the environment of physical abrasion or harsh environments the products are being used in. Hot dip galvanizers commonly apply an excessive amount of zinc weight to their products compared to in-line galvanized manufacturers. The pure zinc layer provides the greatest protection thereby extending the lifetime of a product against corrosion. Continuous galvanizing processes, such as ILG and Zinc-Tech™ require less than half the amount of zinc on application to produce the same thickness of pure zinc resulting in superior protection with savings on zinc.



