Corrosion rate of zinc and steel
Galvanizing is understood as the process of applying a zinc coating to another metal object. Many steel products like tubular and square shapes or sheets used in various fields have been galvanized in order to make the product more resistant against corrosion or rust, which extends their usable lifetime. The galvanizing process is usually performed on the base material steel, as steel has low properties for rust protection and becomes brittle and weak over time.
Let’s try to understand the corrosion rate of zinc and steel:
Test results of iron corrosion rate
To understand the corrosion rate of steel, a composite cycle test that simulates corrosion in different environments without having to wait for years was performed on test pieces in which a split-to-half pipe with outer diameter 31.8mm (1.25in) and a plate of thickness 1.6mm (0.062in) was used without the protective galvanizing layer on the inner surface.
The combined cycle test and the exposure test have the correlation shown in the table below.
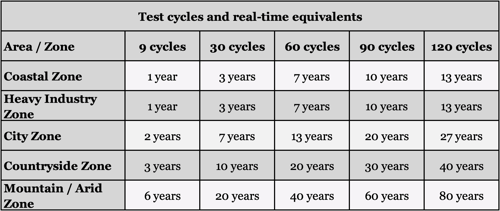
Considering the average corrosion rate in countryside environment (0.014mm/year or 0.0005in/year), we can observe the same with the results of the corrosion test below:
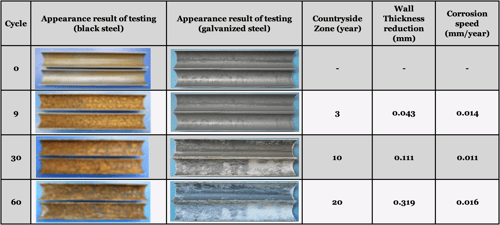
Without the protective zinc coating, steel rusts away rapidly, making it hazardous to use in most applications after just 1-3 years, depending on the environment conditions.
Zinc corrosion rate
The corrosion rate of zinc is much slower than that of steel and the "time to first maintenance" - understood to be when 5% of the steel's surface is rusting - reaches up to 20 years with just a thin galvanizing layer.
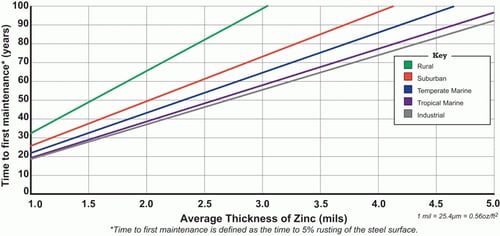
One of zinc's superior corrosion properties come from a dense and thin film of zinc oxide that is formed on the surface which has excellent corrosion resistance and seals the zinc from further corrosion.
Steel corrodes about 0.014mm(0.0005in) / year while zinc corrodes at only 0.0006mm(0.00002in) / year, so steel corrodes about 20 times faster than zinc. In other words, galvanizing extends the lifetime of steel up to 20 times with only minimal coating thickness.
Summary
We explained why zinc has better corrosion resistance properties compared to steel and why it is important for steel to be galvanized in many appliances. To galvanize steel, there are different methods available to manufacturers.
The galvanizing process has been done conventionally by the process of batch hot dip in which the product is manufactured and then “dipped” into a kettle with molten zinc to cover the product with a layer of zinc. In-line galvanizing (ILG) does this in a split-second, requires less space and material, is cost efficient, safe and quality oriented. In replacing the conventional hot-dip process, ILG is capable of galvanizing the products on the existing manufacturing line (tube mill line) without interrupting or stopping the continuous process and providing a finished galvanized product at the end of the manufacturing line.
Contact us to learn more about the process of galvanizing steel tube and its benefits!



